Marina Gabrić Dragović1, Davor Janković1, Nikol Očuršćak1, Máté Hala2, Ferenc Kelemen2
1 Genera Inc. part of Dechra Pharmaceuticals PLC group, Croatia
2 Prophyl Animal Health Ltd, Hungary

Safety evaluation of live vaccines Avishield® IB H120 and Avishield® IB GI-13 against infectious bronchitis when administered to laying hens.
The potential influence of infectious bronchitis virus (IBV) on the reproductive tract of chickens is well established. IBV can replicate in the oviduct causing permanent damage in young hens and the occurrence of false layers. In laying hens, IBV infections can significantly decrease egg production as well as negatively impact egg quality and hatchability.
Objective
Vaccines for use in hens during the production period must have demonstrated safety profile with no interference to egg production.
Here we present the results of two studies examining the safety of live attenuated infectious bronchitis vaccines Avishield IB H120 and Avishield IB GI-13 when administered to chickens inlay
Materials and methods
In the first study, a group of 30 specific pathogen free (SPF) layers were vaccinated twice with Avishield IB H120 vaccine ( Massachusetts serotype ) by spray method.
The first vaccination was at the age of 28 weeks at the peak of lay with a tenfold maximum vaccine dose followed by the second vaccination with a single maximum dose given four weeks later.
In the second study a group of 48 SPF chickens was vaccinated with Avishield IB GI-13 vaccine containing strain V-173/11 (793B serotype), otherwise the same design as in the first study was applied.
During the observation period , the birds were checked daily for general appearance, behaviour and the presence of any IBV-related clinical signs. Eggs were collected daily, counted and examined for the quality grade. Results were compared against the respective unvaccinated control groups.
Vaccination of laying hens with live IBV vaccine strains H120 or V-173/11 = No impact on egg quantity and quality
Results
No clinical abnormalities nor mortality attributable to the IBV vaccine viruses were observed among vaccinated chickens.
The egg production was consistent and comparable showing no statistically significant difference between vaccinated and unvaccinated chickens.
During the observation period of 49 days, 1223 eggs were produced in the H120-vaccinated group (30 birds) compared to 1228 eggs in the control group (30 birds (Figure 1).
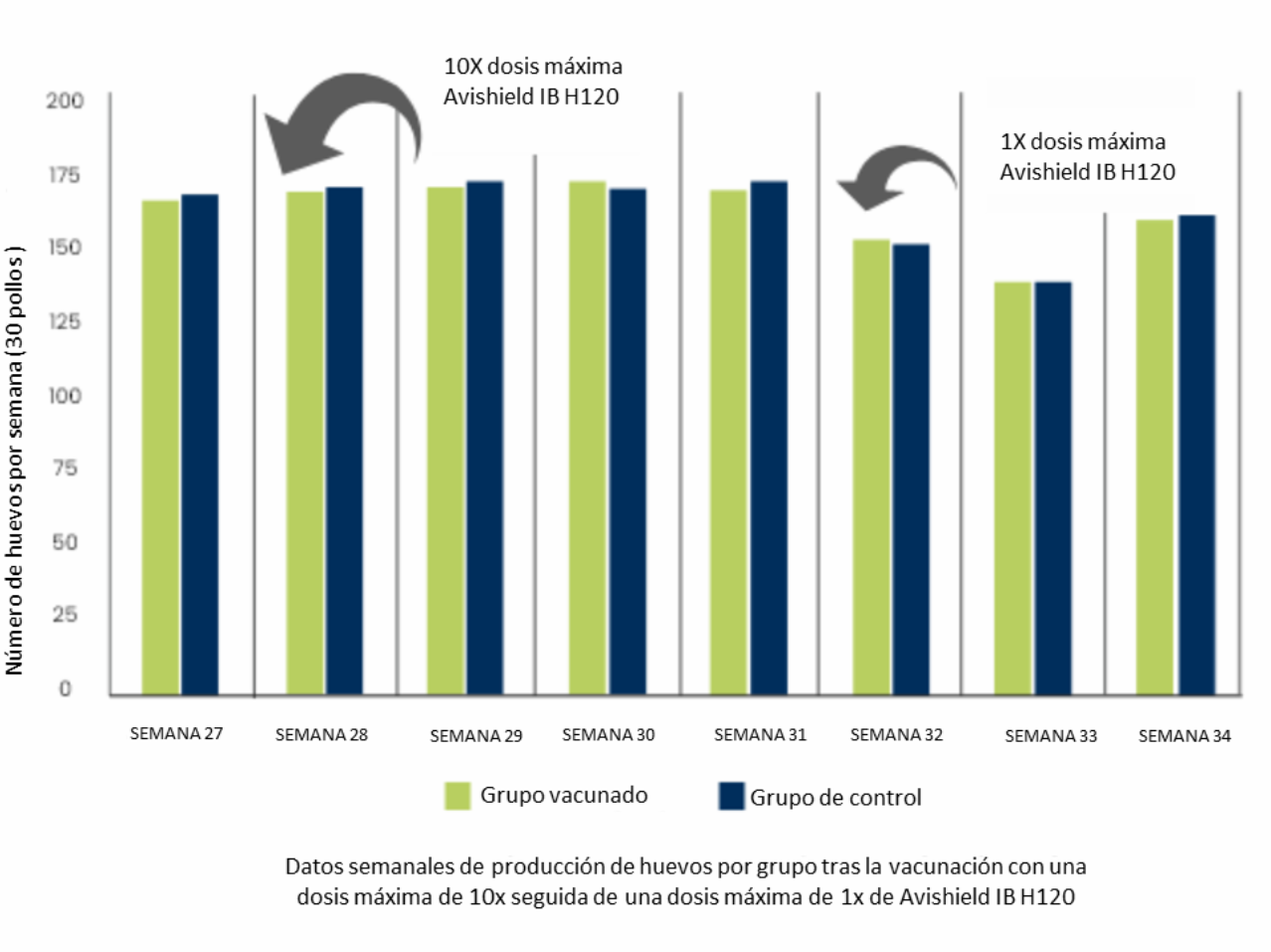
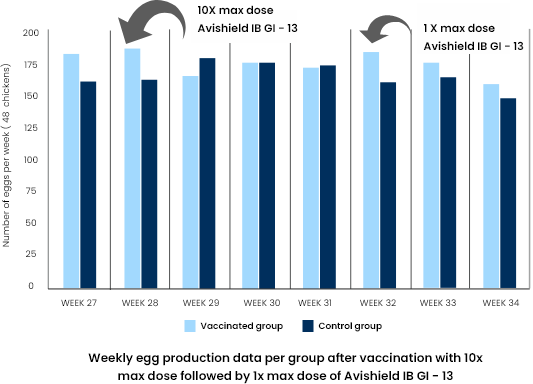
Altered egg quality distribution between the groups after vaccination till end of observation period (week 28-week 34)
| Avishield IB H120 | |||
|---|---|---|---|
| Altered egg quality | Vaccinated group | Control group | |
| No-shell egg | 1 | 0 | |
| Borken/mended shell egg | 0 | 3 | |
| Rounded egg | 0 | 1 | |
| Elongated egg | 1 | 1 | |
| Total | 2 | 5 | |
| Avishield IB GI - 13 | |||
|---|---|---|---|
| Altered egg quality | Vaccinated group | Control group | |
| No -shell egg | 0 | 1 | |
| Very thin shell | 1 | 0 | |
| Small egg | 1 | 4 | |
| Total | 2 | 5 | |
Conclusion
After administration of a tenfold maximum dose followed by one maximum dose of H120 or V-173/11 vaccine:
- Neither vaccine caused clinical abnormalities or mortality in the chickens.
- Egg production and quality were similar between vaccinated and unvaccinated chickens.
- The vaccines have an adequate safety profile for use in chickens during the laying period
References
1 - Jackwood, M. W., J. J. De Wit (2020): Infectious Bronchitis. In: Swayne, D. E. , M. Boulianne, C.M. Logue, L.R. McDougald, V. Nair, D.L. Suarez, S. Wit, T. Grimes, D. Johnson, M. Kromm, T.Y. Prajitno, I. Rubinoff, G. Zavala (Eds). Diseases of Poultry, 14th ed., 167–188.
2 - Gabrić Dragović, M., L. Kutle, L. Špirić, S. Abramović, M. Halas, F. Kelemen (2022): Safety of the live attenuated vaccine Avishield IB GI-13 against infectious bronchitis in SPF chickens during laying period. Proceedings XIV symposium Poultry days, Poreč, Croatia, May 11-14,2022,pp. 71-77.
